Note: This text has been automatically extracted via Optical Character Recognition (OCR) software.
T^
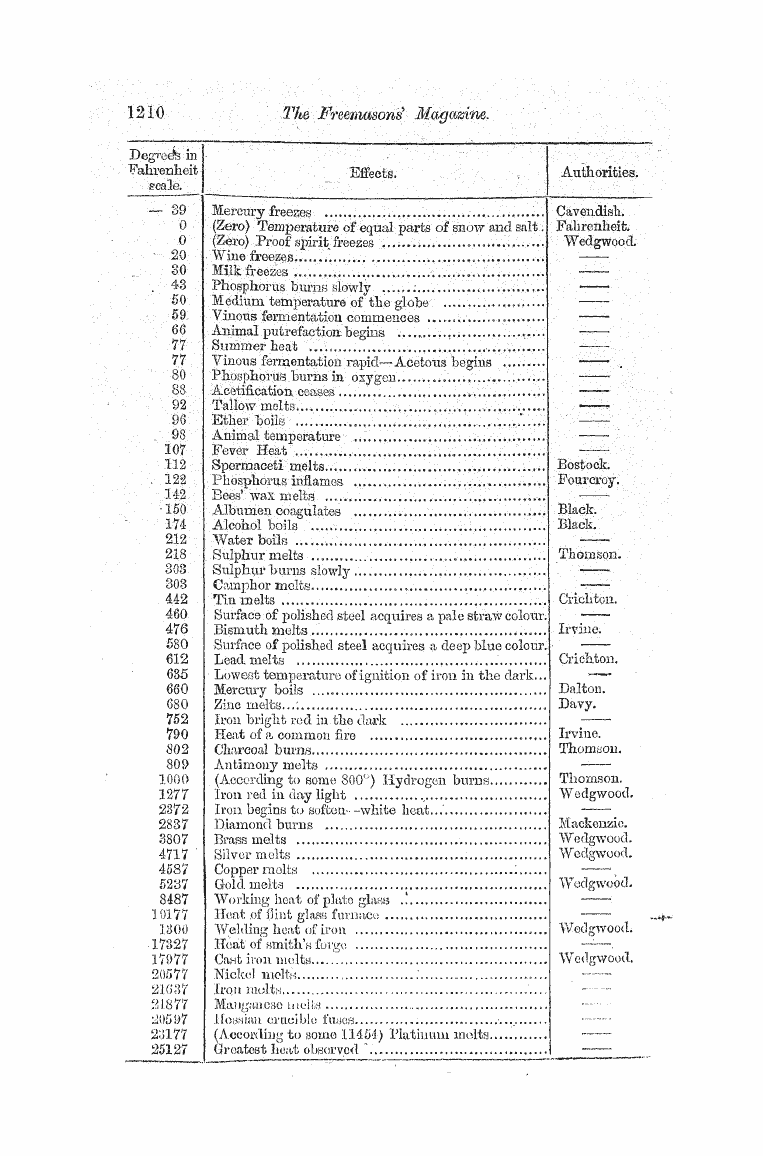
arbitrary , and vary according to the scale used . The scale most commonly in use in England is point at which the top of the column of mercury in the tuhe stands when the bulb is immersed in melting in is marked 32 . If heat be applied to the bulb , the dilatation of the liquid will cause it to rise in the tube , and when the temperature of water is reached it will stand
at a point which is marked 212 . When the mercury stands at intermediate poiiits of the scale , it indicates a temperature in the surrounding objects corresponding with its position on the scale . The space between the freezing and boiling points of water is divided
into 180 parts , each constituting a degree , and the numbering is eontinued at the same rate above and below these points . When the ribrbfri ^ at 0 of the scale , it is said to be I at zero , but this ; expression is perfectly conventional , as we know of no absolute zero of heat . Mercury j freezes at 39 ° , or 39 ° below 2 ; ero . and boils at about 6 60 ° , it can therefore only be used
as a measurement of temperatures betweerr these p oints , iror temperatures below 39 , alcohol is used , which has never be any degree of cold either naturally or artificially produced .. For temperatures above 660 ° , such as the heat of furnaces , ( fee .,
instruments called pyrometers are employed , the principle of which depends generally on the expansion of clay , or some substance unaftected with heat , as compared with that of a metal . 7 . : Theories erf He which have been proposed relative to the nature of heat iliay be divided into two . classes .- In the first it is supposed to be an independent fluid , capable of penetrating , and diffusing itself throughout the pores and interstices of all bodies , solid , liquid , or gaseous , and of being transmitted through space , and
communicated from one body to another . In the other ibis considered to be merely a quality of some other substance , and this quality is commonly assumed to be motion , either in the form of vibration or undulation . The advocates of the former theory generally consider heat to be a material substance , to which they give the name of calorie , or matter of heat . But besides the objection that this matter has never been
obtained in a separate form , there is another , namely , that it does not appear to be subject to that property common to all material bodiesgravitation . The most delicate and careful experiments have foiled to show the slightest increase of weight in bodies after they have been heated , or any loss of weight even when a considerable quantity of latent heat has been disengage ^ There is also another obj ection—it tails to account for the production of heat by friction . When bodies
are rubbed together , no change takes place either in tlio specific heat of the bodies or in tlieir constitution , and yet heat is generated , and continues to be generated without apparent limit . Yet this theory is convenient in explaining many of the phenomena of heat . Latent heat is supposed to arise from a chemical coinbination taking place between the particles of caloric and those of tho body containing it .
Note: This text has been automatically extracted via Optical Character Recognition (OCR) software.
T^
arbitrary , and vary according to the scale used . The scale most commonly in use in England is point at which the top of the column of mercury in the tuhe stands when the bulb is immersed in melting in is marked 32 . If heat be applied to the bulb , the dilatation of the liquid will cause it to rise in the tube , and when the temperature of water is reached it will stand
at a point which is marked 212 . When the mercury stands at intermediate poiiits of the scale , it indicates a temperature in the surrounding objects corresponding with its position on the scale . The space between the freezing and boiling points of water is divided
into 180 parts , each constituting a degree , and the numbering is eontinued at the same rate above and below these points . When the ribrbfri ^ at 0 of the scale , it is said to be I at zero , but this ; expression is perfectly conventional , as we know of no absolute zero of heat . Mercury j freezes at 39 ° , or 39 ° below 2 ; ero . and boils at about 6 60 ° , it can therefore only be used
as a measurement of temperatures betweerr these p oints , iror temperatures below 39 , alcohol is used , which has never be any degree of cold either naturally or artificially produced .. For temperatures above 660 ° , such as the heat of furnaces , ( fee .,
instruments called pyrometers are employed , the principle of which depends generally on the expansion of clay , or some substance unaftected with heat , as compared with that of a metal . 7 . : Theories erf He which have been proposed relative to the nature of heat iliay be divided into two . classes .- In the first it is supposed to be an independent fluid , capable of penetrating , and diffusing itself throughout the pores and interstices of all bodies , solid , liquid , or gaseous , and of being transmitted through space , and
communicated from one body to another . In the other ibis considered to be merely a quality of some other substance , and this quality is commonly assumed to be motion , either in the form of vibration or undulation . The advocates of the former theory generally consider heat to be a material substance , to which they give the name of calorie , or matter of heat . But besides the objection that this matter has never been
obtained in a separate form , there is another , namely , that it does not appear to be subject to that property common to all material bodiesgravitation . The most delicate and careful experiments have foiled to show the slightest increase of weight in bodies after they have been heated , or any loss of weight even when a considerable quantity of latent heat has been disengage ^ There is also another obj ection—it tails to account for the production of heat by friction . When bodies
are rubbed together , no change takes place either in tlio specific heat of the bodies or in tlieir constitution , and yet heat is generated , and continues to be generated without apparent limit . Yet this theory is convenient in explaining many of the phenomena of heat . Latent heat is supposed to arise from a chemical coinbination taking place between the particles of caloric and those of tho body containing it .