Note: This text has been automatically extracted via Optical Character Recognition (OCR) software.
T^
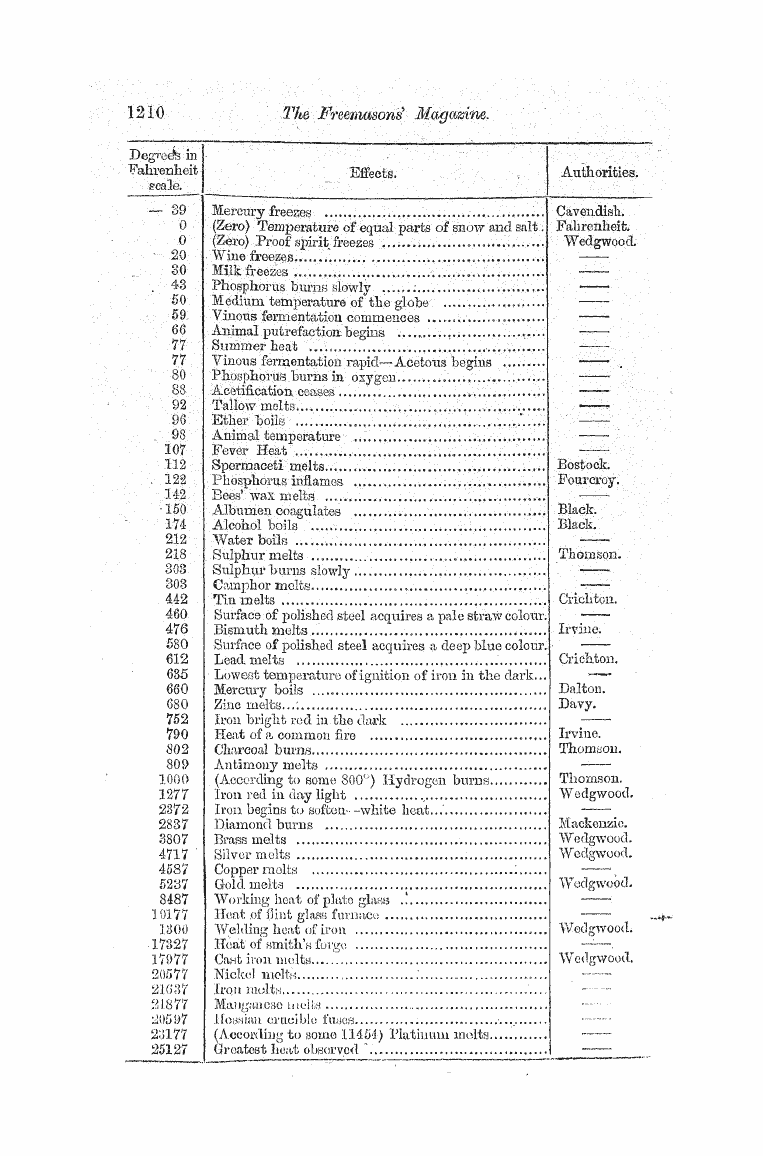
Th < e various capacities of bodies for he ^ affinity existing between calorie and different bodies , similar to that between chemical substances . The expansion consequent upon raising bodies to a higher temperature is supposed to be caused by the introduction of the caloric forcing the particles of the body to a greater
distance apart , thus occasioning aninerease ^ df bulk . The radiation of heat is supposed to be the transmit of particles of caloric in straight lines through space , which are absorbed or reflected oh encountering objects in their course . In this respect the theory is similar to the corpuscular theory of light . In the vibratory theory heat is supposed to be caused by the vibration or undulation , either of the body fe or oif-ar subtle etherial fluid , pervading all space , and similar , if not identical with , the luminiferous ether . The vibrations are transferred foom one bod to another according to the" laws of motion , and the radiation is similar to that of light in the undulatory theory . One of the most
powerful arguments in favour of this theory is furnished by the great similarity existing between the rays of heat and those of light , which We have already adverted to ; and it seems highly probable that the radiation of both light and heat is occasioned by the undulation of the luminiferous ether , the undulations differing only in magnitude and intensity . Some of the phenomena of tliegen ^
especially in the case of friction , may be add vibratory theory , for it is easy to conceive that a body may be put into a state of vibration by certain processes , such as percussion and friction ; but we cannot suppose , in the material theory , that fresh particles of caloric can be actuallv created by such means .
Sir Humphry Davy thus expresses his views in regard to the nature of heat : — u It seems possible to account for all the phenomena of heat , if it be supposed that , in solids , the particles are in a constant state of vibratory motion , the particles of the hottest body moving with the greatest velocity , and through the greatest space * , that in liquids and elastic fluids , besides
the vibratory motion , which must be conceived , greatest m the last , the particles have a motion round their own axes , with different velocities , the particles of clastic fluids moving with the greatest quickness ; and that , in etherial substances , the particles move round their own axes , and separate from each other , penetrating in right lines through space . Temperature may be conceived to be dependent on the velocity of the vibrations ; increase of the capacity on the motion being performed in greater space * , and the
diminution of temperature , during the conversion of solids into liquids or gases , may he explained on the idea of the loss of vibratory motion , in consequence of the revolution of particles round their axes , at the moment when the body becomes liquid or aeriform , or from the loss of rapidity of vibration , in consequence of the motion of the particles through greater space . "
8 . Applications . ' — Certainly the most striking application of the properties of heat is in the great force exerted in the conversion of water into steam by means of heat as a motive power . The illustrious James Watt proved that by adding , to water , at 212 ° , an
Note: This text has been automatically extracted via Optical Character Recognition (OCR) software.
T^
Th < e various capacities of bodies for he ^ affinity existing between calorie and different bodies , similar to that between chemical substances . The expansion consequent upon raising bodies to a higher temperature is supposed to be caused by the introduction of the caloric forcing the particles of the body to a greater
distance apart , thus occasioning aninerease ^ df bulk . The radiation of heat is supposed to be the transmit of particles of caloric in straight lines through space , which are absorbed or reflected oh encountering objects in their course . In this respect the theory is similar to the corpuscular theory of light . In the vibratory theory heat is supposed to be caused by the vibration or undulation , either of the body fe or oif-ar subtle etherial fluid , pervading all space , and similar , if not identical with , the luminiferous ether . The vibrations are transferred foom one bod to another according to the" laws of motion , and the radiation is similar to that of light in the undulatory theory . One of the most
powerful arguments in favour of this theory is furnished by the great similarity existing between the rays of heat and those of light , which We have already adverted to ; and it seems highly probable that the radiation of both light and heat is occasioned by the undulation of the luminiferous ether , the undulations differing only in magnitude and intensity . Some of the phenomena of tliegen ^
especially in the case of friction , may be add vibratory theory , for it is easy to conceive that a body may be put into a state of vibration by certain processes , such as percussion and friction ; but we cannot suppose , in the material theory , that fresh particles of caloric can be actuallv created by such means .
Sir Humphry Davy thus expresses his views in regard to the nature of heat : — u It seems possible to account for all the phenomena of heat , if it be supposed that , in solids , the particles are in a constant state of vibratory motion , the particles of the hottest body moving with the greatest velocity , and through the greatest space * , that in liquids and elastic fluids , besides
the vibratory motion , which must be conceived , greatest m the last , the particles have a motion round their own axes , with different velocities , the particles of clastic fluids moving with the greatest quickness ; and that , in etherial substances , the particles move round their own axes , and separate from each other , penetrating in right lines through space . Temperature may be conceived to be dependent on the velocity of the vibrations ; increase of the capacity on the motion being performed in greater space * , and the
diminution of temperature , during the conversion of solids into liquids or gases , may he explained on the idea of the loss of vibratory motion , in consequence of the revolution of particles round their axes , at the moment when the body becomes liquid or aeriform , or from the loss of rapidity of vibration , in consequence of the motion of the particles through greater space . "
8 . Applications . ' — Certainly the most striking application of the properties of heat is in the great force exerted in the conversion of water into steam by means of heat as a motive power . The illustrious James Watt proved that by adding , to water , at 212 ° , an